
The so-called anti-counterfeiting directive 2011/62 / EU (from the English Falsified Medicine Directive, FMD) has been in force since 9.2.2019. Medicinal products (LPs) manufactured after this date must contain protective elements to verify authenticity, to identify individual packages and to ensure that the packaging has not been tampered with. The security features are a unique identifier and a means of verifying the handling of the packaging. The unique identifier in the form of a 2D code contains the product code (indicating the name, pharmaceutical form, strength, size and type of package), the serial number of the package, the batch number and the expiry date. The means for verifying the handling of the package is usually solved by gluing the outer package (box) so that the package is irreversibly broken after the first opening. The exceptions are most over-the-counter or White List medicinal products (eg allergen extracts, advanced therapy medicinal products…). Packages manufactured before 9.2.2019 are exempted until the end of their shelf life.
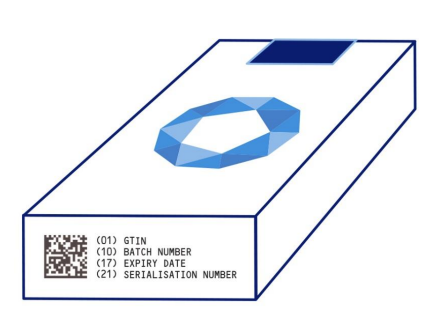
Manufacturers upload data on manufactured pieces to a storage system consisting of a central repository of the European Union and at least one national or transnational repository for each EU Member State. The repository must not allow the upload of a unique identifier that would contain the same product code and the same serial number that already contains another uploaded identifier in the repository. Authentication is performed by manufacturers, distributors and pharmacies. The pharmacist verifies the protective elements at the time of dispensing the medicinal product to the patient. It checks the integrity of the package and by retrieving the unique identifier, the identifier of the specific package is invalidated in the repository. It is therefore necessary to retrieve the identifier of each medicinal product dispensed.

Due to the initial high error rate of the verification system and the absence of counterfeits in pharmacies in the Czech Republic, it was agreed with the Ministry of Health during 2019 that pharmacists will be obliged to verify the dispensed packages of medicinal products. However, in the case of an error message during authentication (so-called alert), it will be at the discretion of the pharmacist whether to dispense the medicinal product. Thus, if there is no reason to suspect that it is a counterfeit, he can give the medicine to the patient.
As of 1 January 2021, the directive is already in full operation and the obligations for the verification of medicinal products must be fulfilled in full. If reading the identifier during dispensing at the pharmacy triggers an error message (alert), it is no longer possible to dispense the medicine. The package is placed in a reserved place, in the so-called quarantine, where it remains for up to 14 days until its authenticity is verified. If a product is issued that has not passed the verification, the operator of the pharmacy faces a fine of up to 2 million crowns from the State Institute for Drug Control.
Used sources: National Organization for the Authentication of Medicines, zs (czmvo.cz)

31. 12. 2025
Antibiotic resistance continues to rise. The eleventh annual World Antibiotic Week, organized by the World Health Organization, took place during November.

30. 11. 2025
The pitfalls of mail order dispensingAt the recent XXXIVth congress of delegates of the Czech Chamber of Pharmacists, the discussion about the possibility of introducing mail order dispensing was reopened ...